Health Canada is reducing the retention period for clinical trial records for drugs and natural health products from 25 years to 15 years.
Continue reading “Health Canada: Period Reduced for Keeping Clinical Trial Records”
Organization Categories: BC AHSN
2022 suite of funding opportunities: contributing to advancing research-based evidence into action
Announcing Michael Smith Health Research BC 2022 suite of research competitions.
REBC collaborates with Indigenous artist to create artwork for the 2022 Cultural Safety and Humility Workshop
The important work of Indigenous cultural safety and humility has come to the forefront of discussions within the research ethics community. Continue reading “REBC collaborates with Indigenous artist to create artwork for the 2022 Cultural Safety and Humility Workshop”
Our Unit Director Talks Clinical Trials with Chatelaine Magazine
In an interview with the Canadian women’s magazine “Chatelaine,” Alison Orth discusses the important role of clinical trials in “finding new ways to detect, diagnose, and treat diseases”. Continue reading “Our Unit Director Talks Clinical Trials with Chatelaine Magazine”
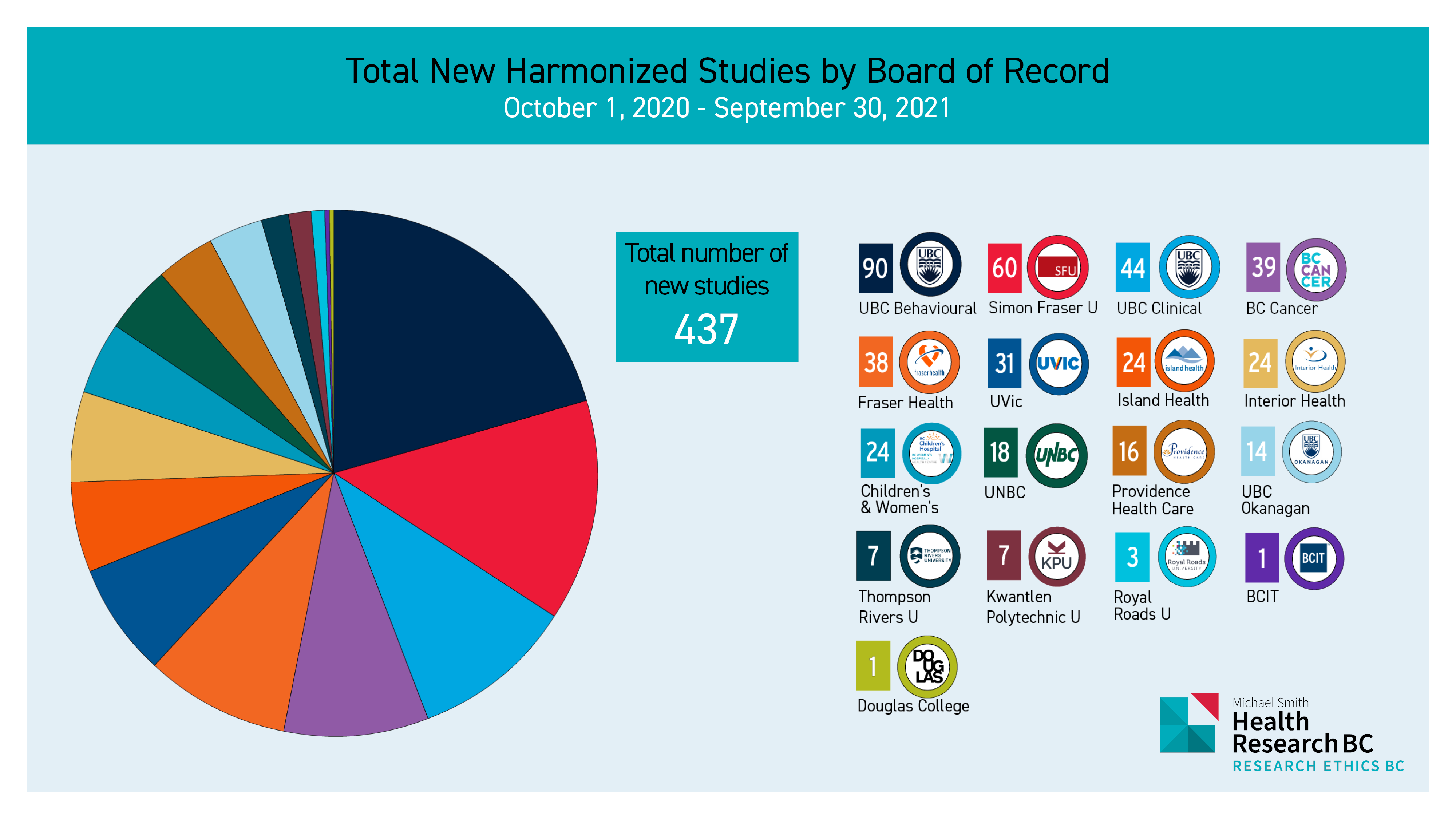
REBC Reports on 2020/2021 Metrics for the Provincial Research Ethics Platform
Research Ethics BC is excited to report on the metrics taken from the Provincial Research Ethics Platform (PREP) for the 2020/2021 year. Continue reading “REBC Reports on 2020/2021 Metrics for the Provincial Research Ethics Platform”
ICH E8(R1) Guideline Reaches Approval
Clinical Trials BC shares that The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has approved the revised version of the primary efficacy document known as ‘General Considerations in Clinical Trials’. Continue reading “ICH E8(R1) Guideline Reaches Approval”
A 20-year journey of health research in BC: Voices for patient-oriented research
Patient partners help researchers see things they may not have thought of.
Continue reading “A 20-year journey of health research in BC: Voices for patient-oriented research”
REBC thanks their provincial partners
REBC wants to demonstrate our sincerest gratitude for the continued dedication of all the research ethics provincial partners that have facilitated ongoing research and BC’s researchers.
A 20-year journey of health research in BC: Streamlining the ethics review process
In 2010, funded by a $1 million grant, the BC Ethics Harmonization Initiative (BCEHI) was born. Its goal was simple but complex…
Advancing Pragmatic Clinical Trials to Support Learning Health Systems: Opportunities for BC
We brought together key partners at Clinical Trials BC’s Modernization of Clinical Trials symposium to discuss how to enhance capacity for pragmatic clinical trials in the BC health system landscape.