REBC Reports on 2020/2021 Metrics for the Provincial Research Ethics Platform
17 November 2021
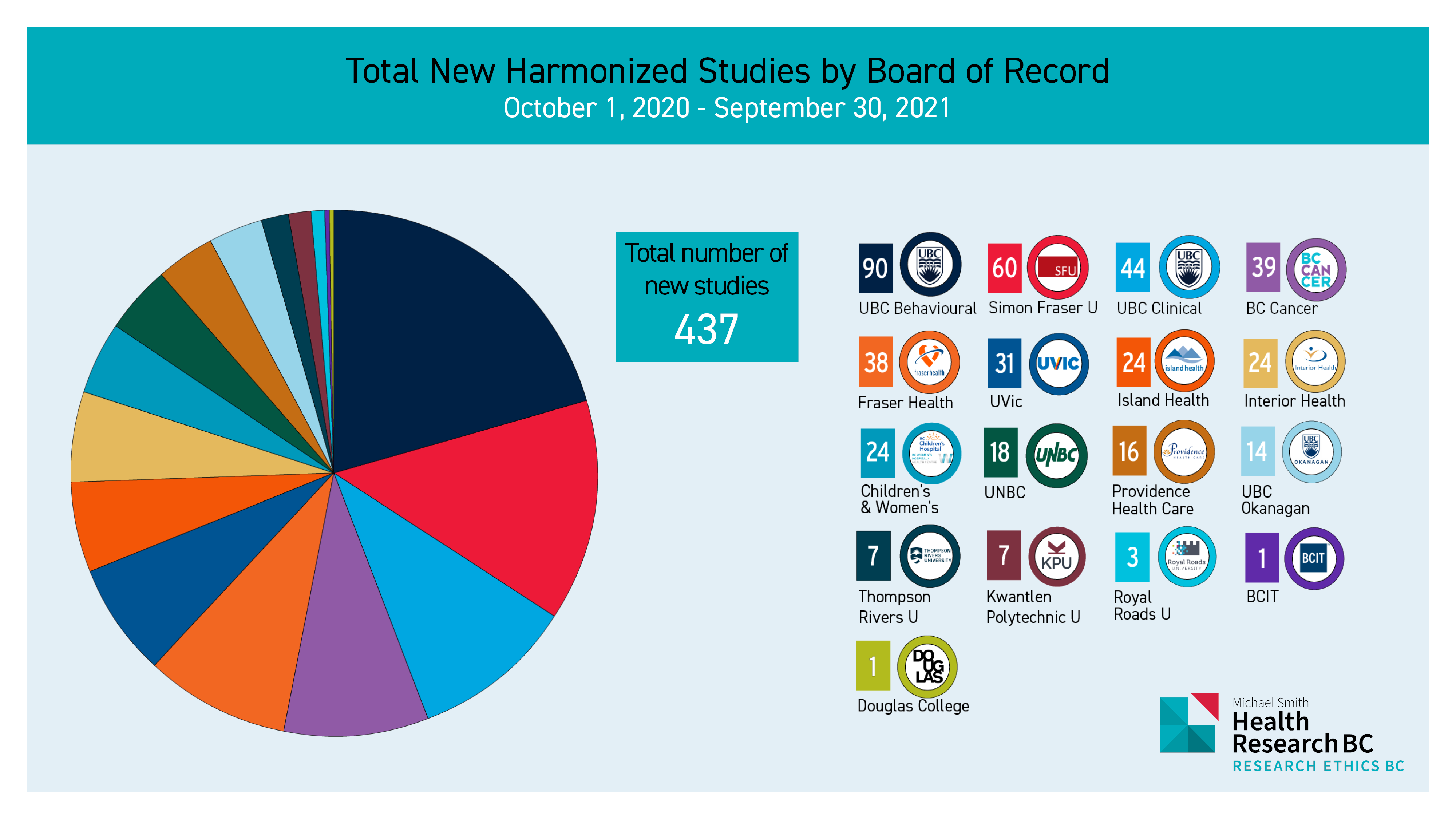
Research Ethics BC is excited to report on the metrics taken from the Provincial Research Ethics Platform (PREP) for the 2020/2021 year. These charts showcase another year of continued growth across the provincial research landscape. We wish to take this opportunity to recognize all the incredible work of the Research Ethics Board (REB) members and REB administrators who work to improve the efficiency and quality of review for the ethical conduct of research involving participants in multi-jurisdictional research studies.
As the above pie chart demonstrates, 437 new harmonized research ethics submissions were approved across the REBC network. Of those 437 new studies, the Board of Record or BoR who is lead REB for the harmonized ethics review, the UBC Behavioural REB, SFU REB, and UBC Clinical REB represented 20.5%, 14%, and 10% respectively, of the total number of newly approved harmonized studies by BoR.
Table 1.1: New harmonized research ethics submissions in PREP by year
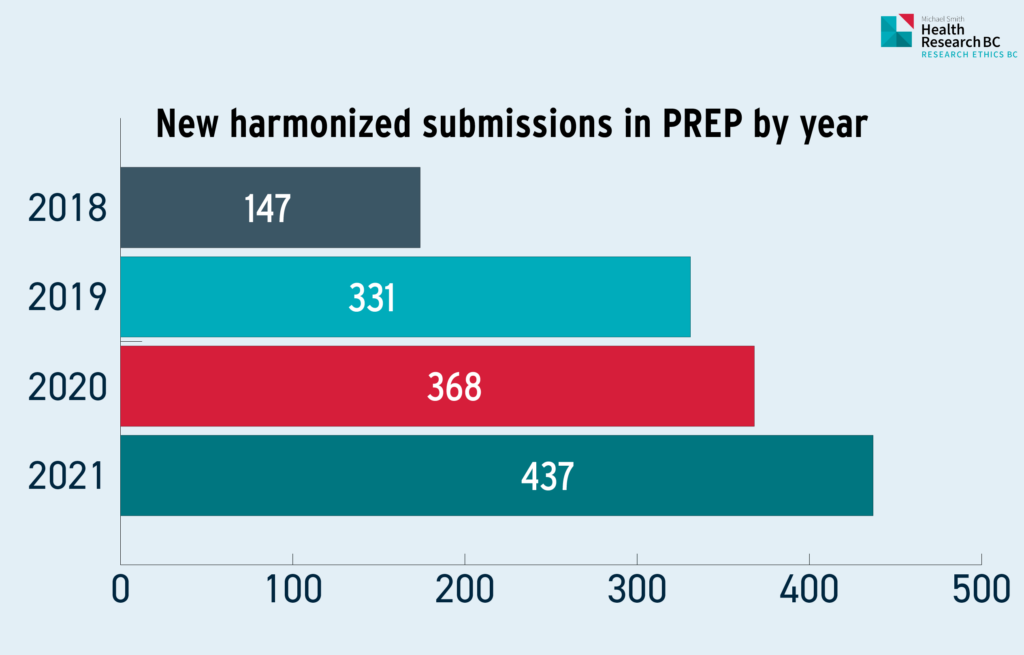
Table 1.1 represents the total number of new harmonized submissions from the period between October 1, 2020 to September 30, 2021. This number does not represent ongoing research studies that have been renewed and/or new amendments processed for harmonized studies.
Table 1.2: Total number of harmonized studies in PREP – New & Active
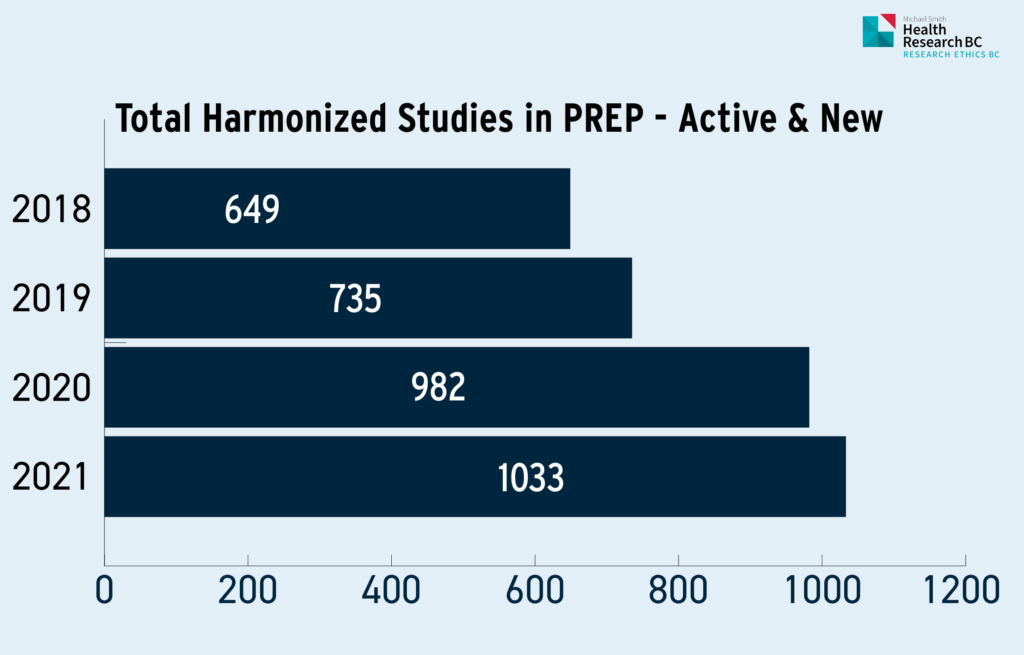
The bar graph table 1.2 represents the total number of active and new harmonized studies. Active harmonized studies are ongoing studies that renew their ethics approval each year through PREP as well as ongoing studies which often will include amendments to the original study protocol.
Table 1.3: Breakdown of Approved Harmonized Ethics Reviews
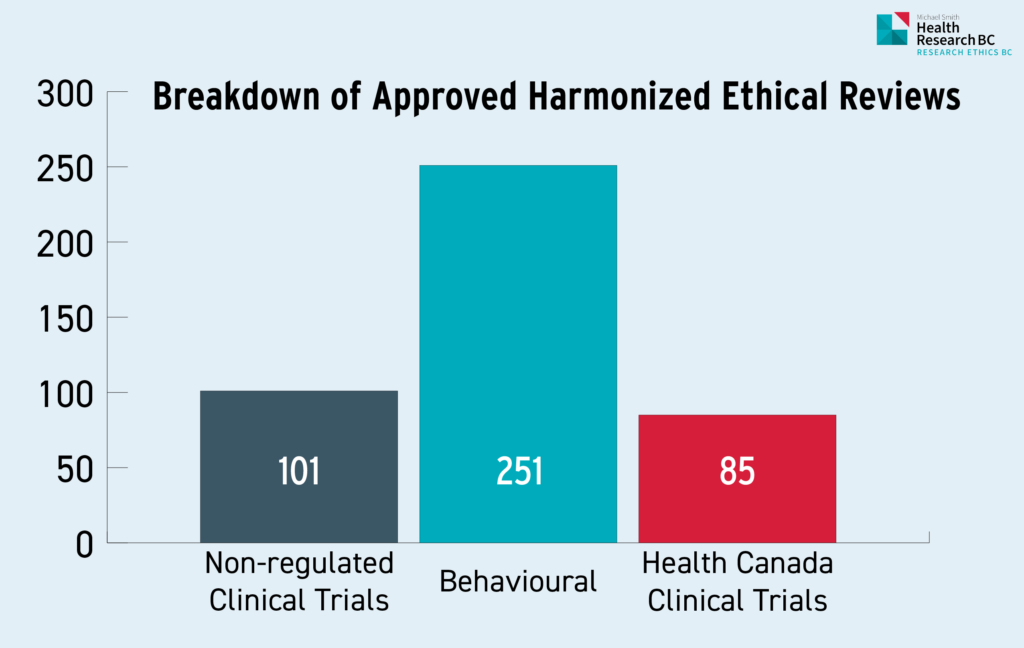
Table 1.3 represents the breakdown of all 437 newly approved harmonized studies this year, with behavioural studies making up the largest proportion with 251 studies or 57% of all harmonized approvals, followed by 101 non-regulated clinical trials (23%) and finally, 85 Health Canada regulated clinical trials made up 20% of all newly approved harmonized studies.
The REBC Network of partners who use PREP for the review of harmonized research studies has shown substantial growth over the last year and since the formal launch of PREP in 2018. For more information or questions relating to PREP metrics please contact ppintovidal@healthresearchbc.ca





