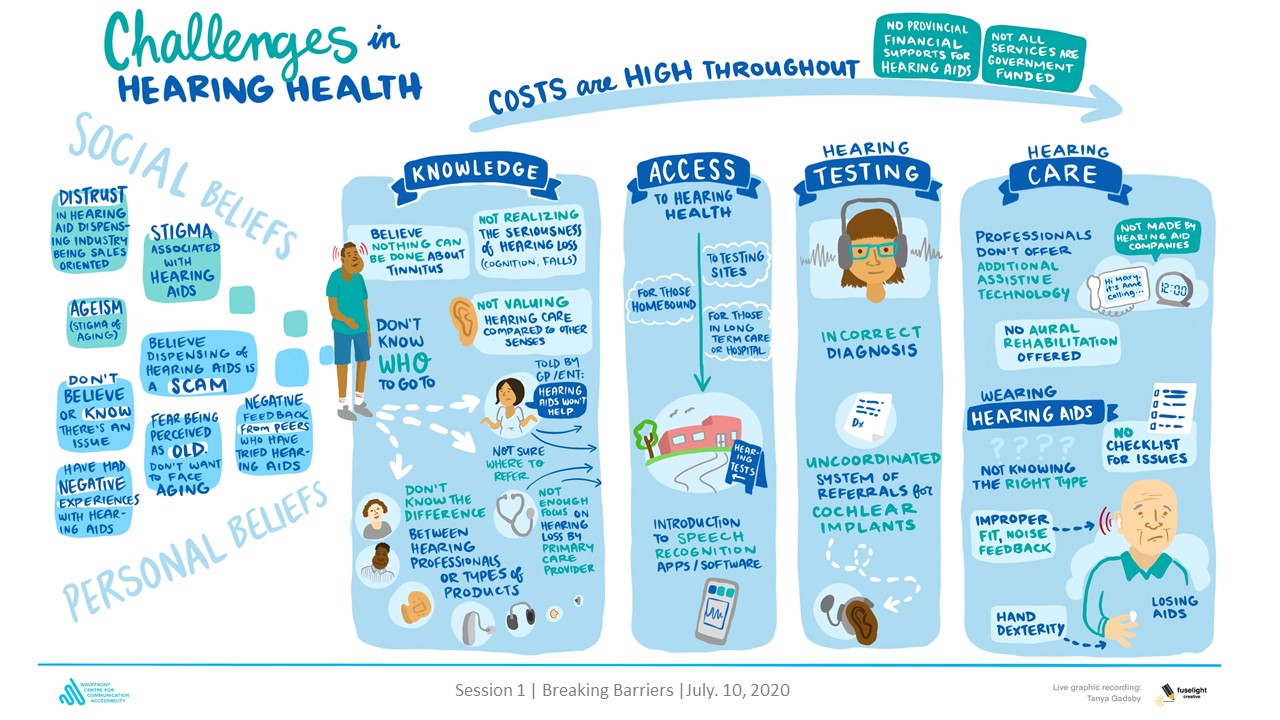
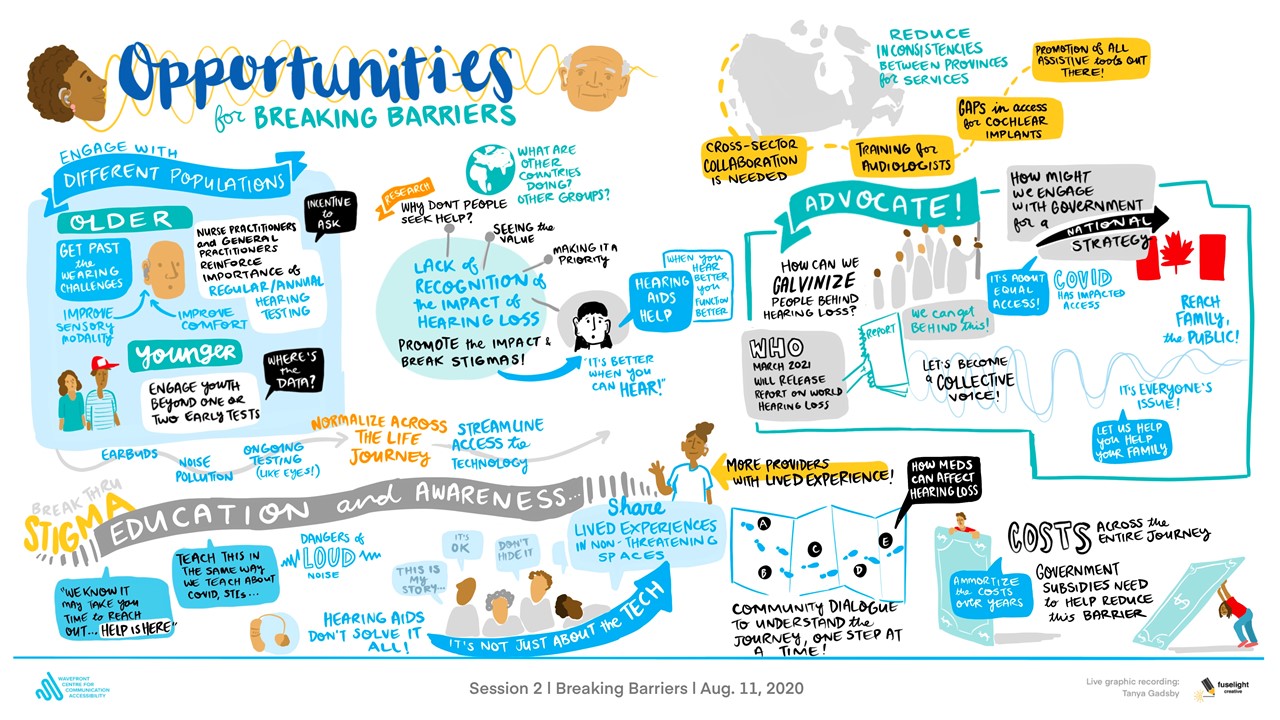
Health Research BC is providing match funds for Phase 2 of the Network, which is funded by the Canadian Institutes of Health Research’s (CIHR) Strategy for Patient-Oriented Research (SPOR) Networks in Chronic Disease.
Supported through CIHR’s Strategy for Patient-Oriented Research and 15 generous funding partners, CHILD-BRIGHT is a pan-Canadian patient-oriented research (POR) network that focuses on brighter futures for children with brain-based developmental disabilities (BDD) and their families.
Dr. Dan Goldowitz, Professor Emeritus of Medical Genetics at UBC and senior scientist at the Centre for Molecular Medicine and Therapeutics at the BC Children’s Hospital Research Institute, is one of CHILD-BRIGHT’s three co-PIs, along with Drs. Steven Miller at UBC, and national PI Annette Majnemer at McGill University.
Our national Network of 350 researchers, clinicians, decision-makers, youth and parents have co-created a novel research program based on priorities identified by patient-partners and other stakeholders that incorporates new technologies and interventions to advance health practice and policy. In our initial phase (2016-22), thirteen projects focused on early intervention that promotes brain/child development, strategies that support mental health of children/families, and service delivery redesign that addresses gaps in service.
A major focus for our Network is moving research into action through insight, inclusivity and methods grounded in implementation science (IS) and knowledge mobilization (KM). In Phase 2 (2022-26), we will accelerate the uptake and use of the knowledge generated in Phase 1 to enhance child health and family well-being within BC and across Canada. Our goal is to become a movement for change by moving patients and families into research teams, moving research into practice and policy, and ultimately moving children and families forward to brighter futures.
Dr. Goldowitz is co-leading CHILD-BRIGHT’s Training and Capacity-building Program with implementation scientist Dr. Celia Laur from the University of Toronto. In concert with his UBC team, they will engage with relevant groups including patients and families, trainees, and researchers to build a comprehensive training program that will enhance core competencies in POR, KM, IS, and EDI.
In addition, a core Phase 2 project spearheaded by Drs. Hal Siden and Tim Oberlander will receive funding support. “Pain and Irritability of Unknown Origin (PIUO): Implementing a Diagnostic Pain Pathway in Community Clinics” will translate the findings from Phase 1 into a readily available tool that can help community pediatricians systematically assess the potential root causes of pain and irritability in children with complex medical needs