Why are pragmatic clinical trials important for our health system?
24 June 2021
First off, what are pragmatic clinical trials?
Most people generally know what clinical trials are: scientific studies that compares health interventions, like different drugs, to study their effects.
What’s less well understood is that very specific conditions need to be met to conduct a clinical study (e.g., controlling the settings in which the interventions are administered, who is recruited to the study, etc).
Given these very specific conditions, do we know if the results of a study done in these conditions will translate to the real world?
Well, we don’t.
Download a screen-reader-friendly PDF of this infographic.
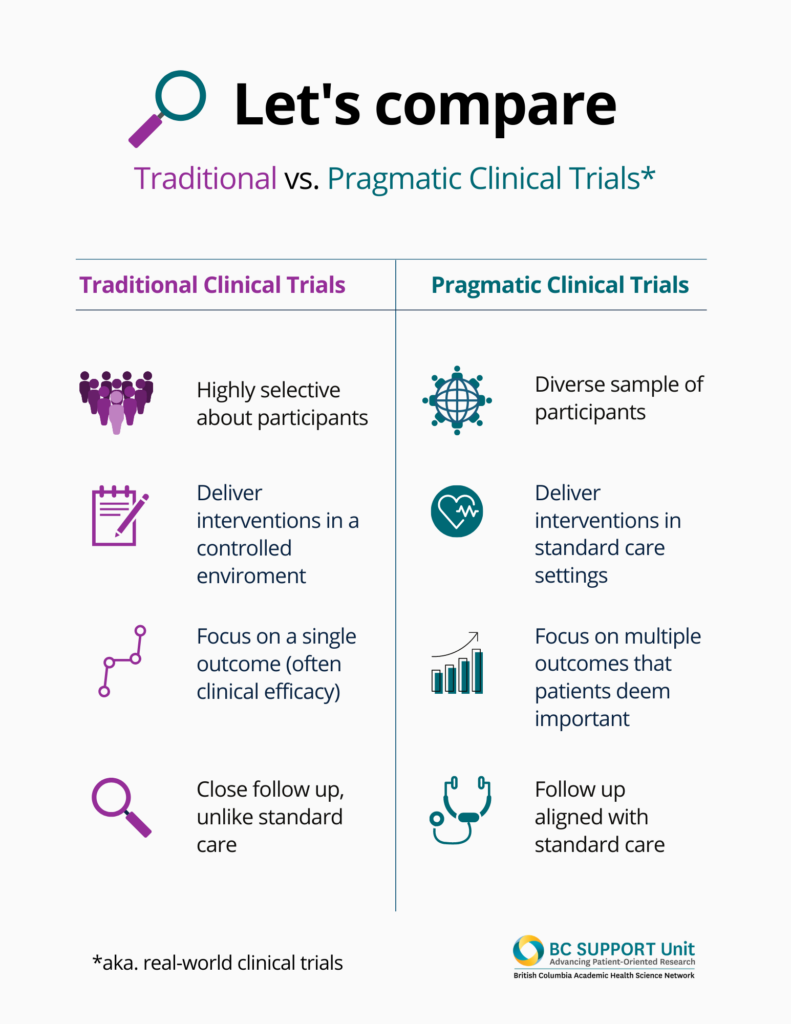
But pragmatic clinical trials or real-world clinical trials aim to bring more real world conditions into the study sphere.
Pragmatic trials study broad patient populations, deliver interventions in usual care settings using minimal extra resources, and evaluate multiple outcomes that are important to patients.
Done well, pragmatic trials can be more person-centred than traditional clinical trials. By focusing on outcomes that patients value, they put the experience of living with health conditions, and undergoing various treatments, at the heart of the study.
Further, pragmatic trials can practice diversity and inclusion in important ways that address health inequities. Clinical trials rely on recruiting volunteers to participate in the study. We know that, for many reasons including the strict inclusion criteria for these studies, clinical trial participants have not reflected the diverse populations that trial results should serve. As a result, clinical trial evidence cannot be safely generalizable across populations already marginalized by gender, race, class, sexual orientation, disability, age, among many other determinants of health. Pragmatic trials attempt to better fill evidence gaps by aiming participant recruitment more broadly. Pragmatic trials not only ‘accept’ diverse participants; embracing, facilitating, and meaningfully considering diversity is one of its strengths.
Sounds great, let’s do it then!
There’s still lots to learn about how to conduct pragmatic trials effectively in real world settings. Research institutions and health institutions are complex organizations of humans, history, and processes; and just doing new things isn’t quite so simple.
So how do we approach embedding pragmatic trials into our health system?
We decided the question was best asked in the context of where we see BC’s health systems moving: learning health systems.
Download a screen-reader friendly PDF of this infographic.
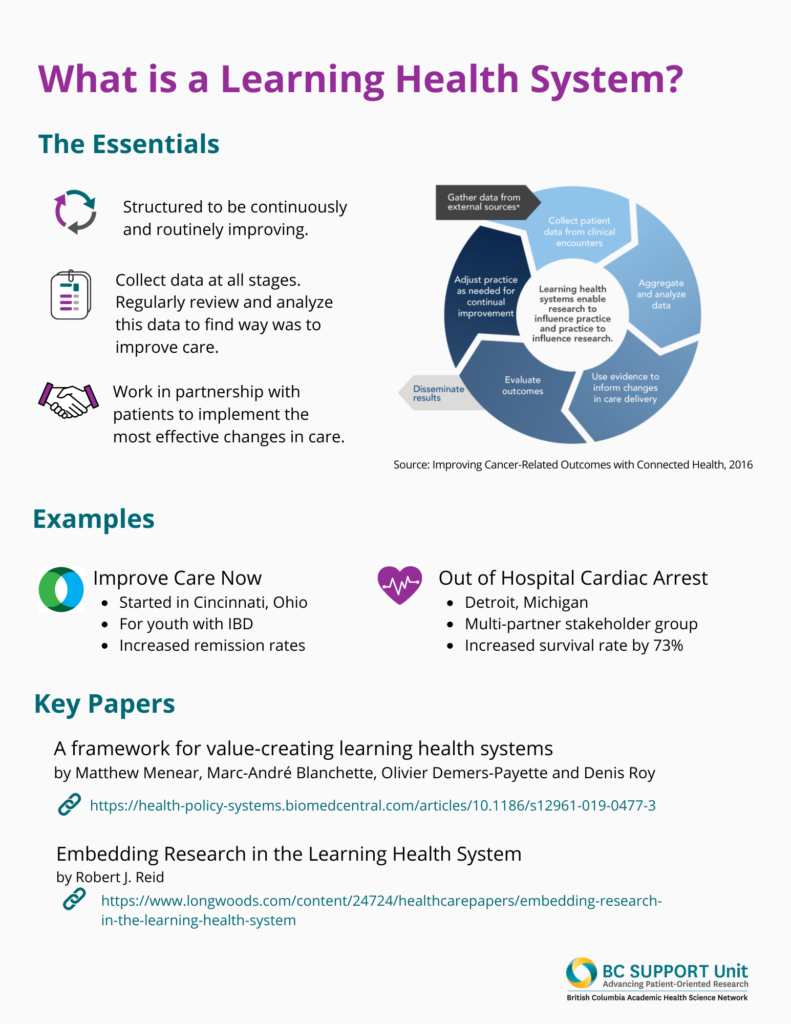
Learning health systems embed processes for collecting and analyzing data created as part of care with the intent to continuously improve care. Put simply, learning health systems embed research in health care. Through quality improvement and research methodologies, these systems can generate new evidence to address knowledge gaps and provide directions for decision-making.
Learning health systems could use pragmatic clinical trials to do this work of collecting data, analyzing data, and improving care. Pragmatic clinical trials support systematic evaluation for how different treatments or ways of delivering care compare. When designed well, they build on existing workflows and data infrastructures to allow the system to ‘learn’ alongside health care delivery. Further, the outcomes assessed are determined by the people for whom the evidence is intended to inform – patients, clinicians, healthcare administrators, and policymakers.
While simple in concept, the design and conduct of a pragmatic clinical trial is not a small undertaking. It requires strong partnership between research and healthcare systems; collaboration with stakeholders, patients, care partners, and family members; and, the presence of an environment and infrastructure to facilitate study conduct.
And so, we brought the people potentially involved in these collaborations, partnerships, environments, and infrastructure together to discuss how to seed the ground to grow pragmatic trials in our health system.
Bringing people together
We heard from our discussions:
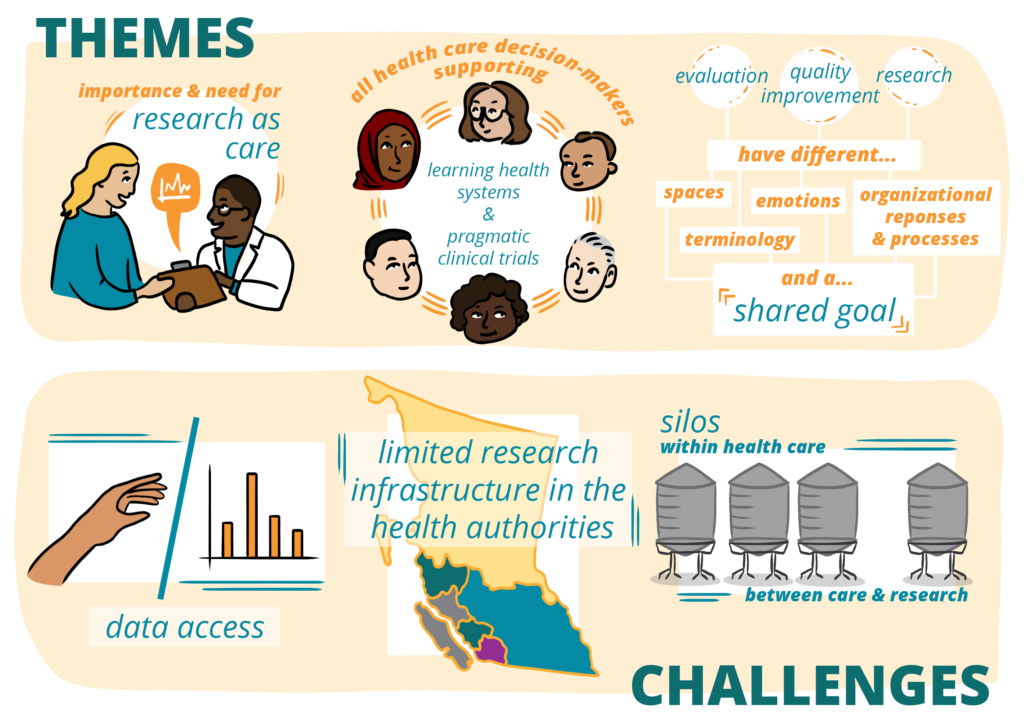
- The importance of and need for embedded research; research as care
- The importance for all health care decision-makers (e.g.., patients, clinicians, administrators) in supporting the advancement of learning health systems and pragmatic clinical trials
- The recognition that while research, quality improvement, and evaluation have common goals, they live in separate spaces, ‘speak’ different languages, evoke different emotions/organizational responses, and follow separate processes for conduct.
Download a screen-reader friendly PDF of this infographic.
We also heard about a number of challenges to embedding pragmatic trials including:
- Data access
- Limited capacity and infrastructure for the conduct of research within health authorities
- Silos that exist both within healthcare and between healthcare and research
Together, we can advance learning health systems, and bridge healthcare and research with pragmatic clinical trials. We look forward to addressing the opportunities and barriers identified in this work, and continuing the conversation for future success in BC.
Read the full report from the conversation.
The Methods Clusters project studied the way that patient-oriented research is done, and how it could be better. In a series of blog posts, team members wrote about their work, what they learned and the best ways to engage patients in health research design. The BC SUPPORT Unit provided funding for the project.
This blog post was written by Hubert Wong.





