Our Impact
Research supports better health.
We support better research.
We help BC researchers launch careers, create life-changing innovations, and attract millions in additional research investment.
We also support system change. We strengthen clinical trials and research ethics, advance priority research on urgent health issues, and expand funding opportunities through strong partnerships.
Since 2001
3,000
researchers supported
since 2001
450
partnerships
Since 2014
$80M
co-invested in research with partners
Since 2001
$280M
invested in Scholars and Research Trainees
Featured CONTENT
Our impact
Research supports better health. We support better research.
Since 2001
3,000
researchers supported
since 2001
450
Partnerships
since 2014
$80M
co-invested in research with partners
since 2001
$280M
invested in scholars and research trainees
We help BC researchers launch careers, create life-changing innovations, and attract millions in additional research investment.
We also drive system change. We strengthen clinical trials and research ethics, advance priority research on urgent health issues, and expand funding opportunities through strong partnerships.
Featured CONTENT
-
Why “research that matters” is grounds for optimism
Learn MoreOur former Chief Scientific Officer Dr. Stirling Bryan shares how priority-based funding ensures a purposeful path forward in tackling urgent health challenges.
-
Walking together: Harley Eagle reflects on health and healing
Learn MoreHarley Eagle, Indigenous Cultural Safety Advisor for Health Research BC, offers insights on how health professionals and researchers can build respectful partnerships with Indigenous communities.
Our Work
Enabling research with impact
We create opportunities for research careers to develop and thrive, connect evidence to the real world, and partner to improve how research is done. By investing in people and strengthening the system in which they work, we enable discoveries that transform health.
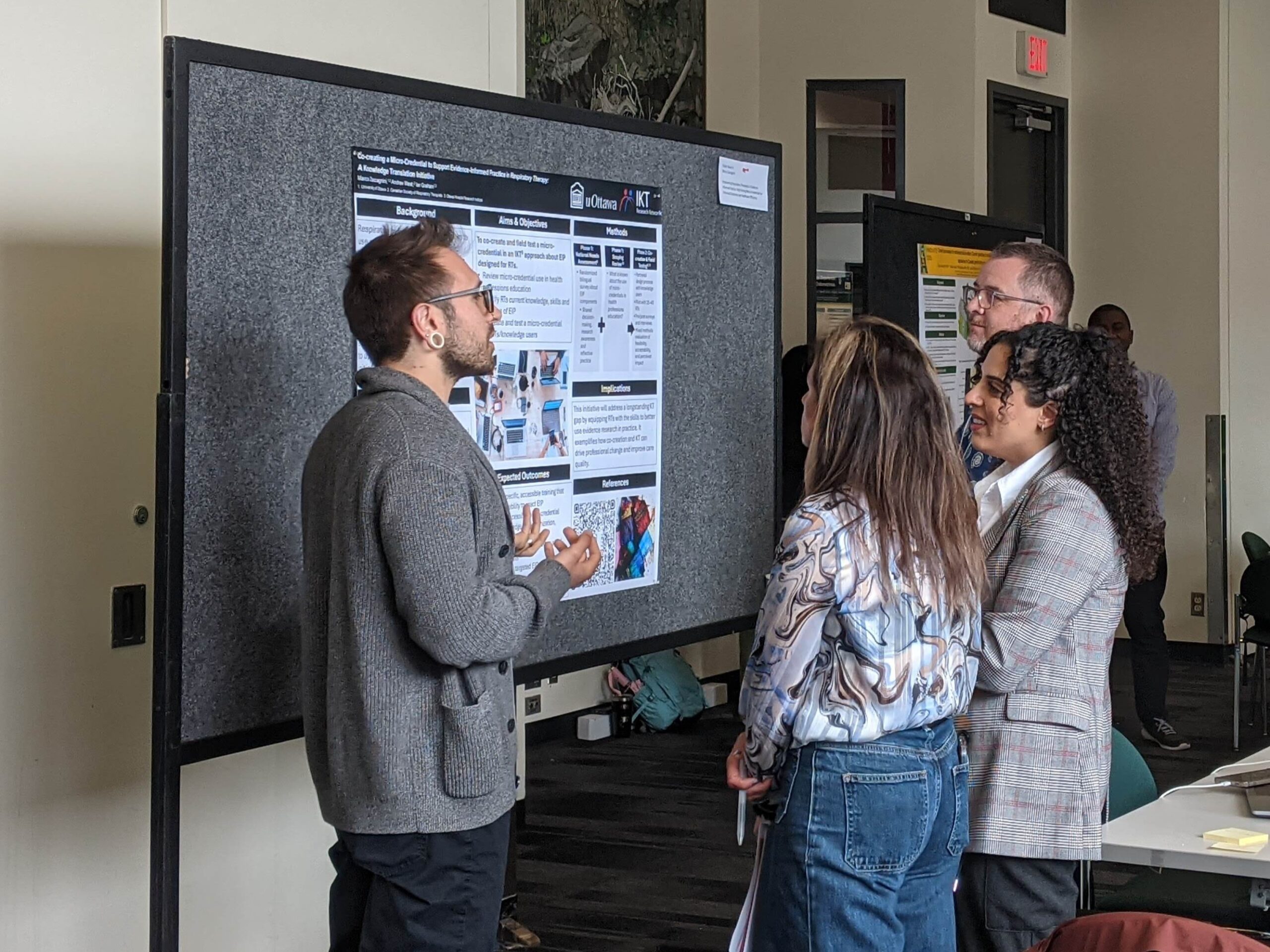
We support a diverse health research workforce across BC.
We connect and align efforts to improve how research is done and used.

We fund research to address pressing health issues.
Partner with us
We amplify research impact through partnership
We work with a wide range of partners to support BC health research talent, build research capacity, and improve the health of British Columbians.
Partnering with us helps organizations maximize their health research investment. We match partner contributions dollar for dollar, effectively doubling the impact and increasing the overall number of awards granted to BC researchers.
Partner with usAbout Us
We enable discoveries that improve lives and strengthen communities
Funded by the provincial government, we support a health research system that drives better health and a stronger economy.
We fund people and research, and we partner on shared opportunities. Our programs build research careers, our investments advance priority research, and our expertise accelerates the use of research evidence.
Get to know usResources
Discover resources and tools to strengthen your research
Access guides, training, and practical tools from Health Research BC. The resources support researchers to strengthen research quality, impact, and collaboration across the province.
Explore resourcesNews & Events
Stay up to date
Explore the latest news from Health Research BC, highlighting discoveries, partnerships, and opportunities that are transforming health across British Columbia.
More stories-
Why “research that matters” is grounds for optimism
Read moreOur former Chief Scientific Officer Dr. Stirling Bryan shares how priority-based funding ensures a purposeful path forward in tackling urgent health challenges.
-
Health Research BC invests $6.3 million to strengthen seniors’ home and community care across British Columbia
Read moreFive research teams across British Columbia are leading projects designed to help seniors age comfortably at home, stay connected, and receive high-quality, evidence-informed care.
Connect with us
We love to chat about our programs, activities and partnership opportunities. Don’t hesitate to reach out to us.
Contact us





